Connectivity of Neuronal Microcircuits
For the function of a given neuron (type) to be accurately assessed, it is essential to map the pre- and post-synaptic partners of that cell (type). We use retrograde markers, such as fluorescent-dye-conjugated Cholera Toxin Beta subunit (CTB), as well as retrograde & anterograde Adeno-Associated Viruses (AAV) of various serotypes, and Canine Adeno-Virus type 2 (CAV2) to fluorescently label neuronal populations. We obtain further specificity by using selective viral promoters, or Cre-recombinase-expressing transgenic mice, to target specific neuronal sub-populations based on their neurochemical identity or post-synaptic target area. Further, these viruses can also be used to simultaneously express genetically-encoded light-controlled ion channels (opsins) to activate or inhibit these different cell types, either in vivo or in vitro, enabling analysis of both structural and functional connectivity. We also investigate the presynaptic inputs to different cell types using monosynaptic rabies virus tracing, allowing us to assess neuronal sub-type-dependent connectivity motifs between identified pre- and post-synaptic neurons in the brain.
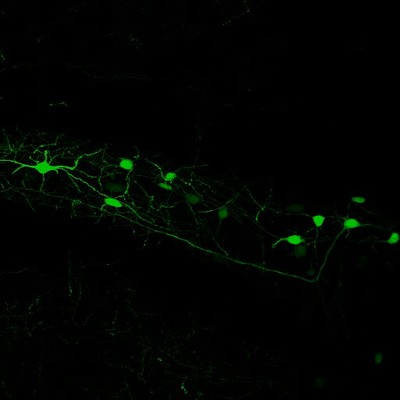
Neurons in the Hilar region of the Hippocampus labelled by injection of an AAV expressing Green Fluorescent Protein (GFP).
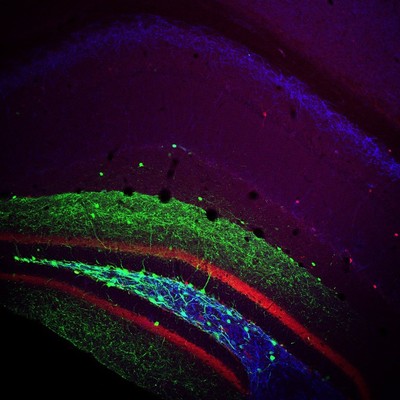
Injection of a cre-dependent virus expressing GFP (green) into the Dentate Gyrus of a transgenic mouse expressing cre under the Somatostatin promoter. Post-hoc immunolabelling for Calbindin (red) and mGluR1a (blue) was also performed.
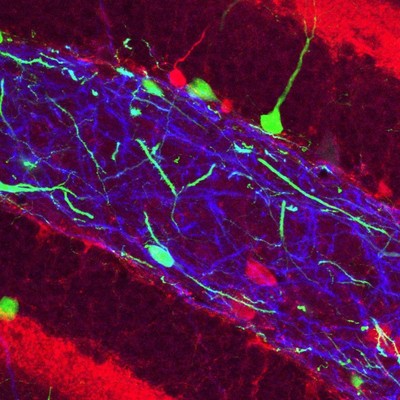
An enlarged view of the Hilar region.

